JARDIANCE is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure.
JARDIANCE is indicated to reduce the risk of sustained decline in eGFR, end-stage kidney disease, cardiovascular death, and hospitalization in adults with chronic kidney disease at risk of progression.
JARDIANCE is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease.
JARDIANCE is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus.
JARDIANCE is not recommended: to improve glycemic control in patients with T1D, it may increase their risk of diabetic ketoacidosis; to improve glycemic control in patients with T2D with an eGFR <30 mL/min/1.73 m2, it is likely to be ineffective based upon its mechanism of action; for treatment of CKD in patients with polycystic kidney disease or patients requiring or with a recent history of IV immunosuppressive therapy or >45 mg of prednisone or equivalent for kidney disease, it is not expected to be effective in these populations.
JARDIANCE is contraindicated in patients with a hypersensitivity to empagliflozin or any of the excipients in JARDIANCE, reactions such as angioedema have occurred.
Scroll below for additional Important Safety Information.
Prescribing Information and Medication Guide
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: Hypersensitivity to empagliflozin or any of the excipients in JARDIANCE, reactions such as angioedema have occurred.
WARNINGS AND PRECAUTIONS
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: JARDIANCE increases the risk of life-threatening ketoacidosis in patients with type 1 diabetes and fatal ketoacidosis has occurred with JARDIANCE. Type 2 diabetes and pancreatic disorders are also risk factors for ketoacidosis and fatal events of ketoacidosis have been reported in patients with type 2 diabetes using JARDIANCE. Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include under-insulinization due to insulin dose reduction or missed insulin doses, acute febrile illness, reduced caloric intake, ketogenic diet, surgery, volume depletion, and alcohol abuse. Signs and symptoms of diabetic ketoacidosis are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Assess patients who present with signs and symptoms of metabolic ketoacidosis, regardless of blood glucose levels. If suspected, discontinue JARDIANCE, treat promptly and monitor for resolution before restarting. Consider ketone monitoring in patients with type 1 diabetes mellitus as well as in others at risk for ketoacidosis. Withhold JARDIANCE in clinical situations known to predispose to ketoacidosis and resume when clinically stable. Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue JARDIANCE and seek medical attention immediately if signs and symptoms occur.
Volume Depletion: JARDIANCE can cause intravascular volume depletion which may manifest as symptomatic hypotension or acute transient changes in creatinine. Acute kidney injury requiring hospitalization and dialysis has been reported in patients with type 2 diabetes receiving SGLT2 inhibitors, including JARDIANCE. Before initiating, assess volume status and renal function in patients with impaired renal function (eGFR <60 mL/min/1.73 m2), elderly patients or patients on loop diuretics. In patients with volume depletion, correct this condition before initiating JARDIANCE. After initiating, monitor for signs and symptoms of volume depletion and renal function.
Urosepsis and Pyelonephritis: Serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization have been identified in patients receiving JARDIANCE. Treatment with JARDIANCE increases the risk for urinary tract infections. Evaluate for signs and symptoms of urinary tract infections and treat promptly.
Hypoglycemia: In adult patients, the use of JARDIANCE in combination with insulin or insulin secretagogues can increase the risk of hypoglycemia. In pediatric patients aged 10 years and older, the risk of hypoglycemia was higher with JARDIANCE regardless of insulin use. The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin.
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases requiring urgent surgical intervention have occurred in both females and males receiving JARDIANCE. Serious outcomes have included hospitalization, multiple surgeries and death. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment and discontinue JARDIANCE.
Genital Mycotic Infections: JARDIANCE increases the risk for genital mycotic infections, especially in patients with prior infections. Monitor and treat as appropriate.
Lower Limb Amputation: Lower limb amputations have been observed in patients with chronic kidney disease taking JARDIANCE. Peripheral artery disease, and diabetic foot infection (including osteomyelitis), were the most common precipitating medical events leading to the need for an amputation. The risk of amputation was highest in patients with a baseline history of diabetic foot, peripheral artery disease (including previous amputation) or diabetes. Counsel patients receiving JARDIANCE about the importance of routine preventative foot care and monitor for signs and symptoms of diabetic foot infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and institute appropriate treatment.
Hypersensitivity Reactions: Serious hypersensitivity reactions have occurred with JARDIANCE (angioedema). If hypersensitivity reactions occur, discontinue JARDIANCE, treat promptly, and monitor until signs and symptoms resolve.
MOST COMMON ADVERSE REACTIONS (≥5%): Urinary tract infections and female genital mycotic infections.
DRUG INTERACTIONS:
Diuretics: Coadministration with diuretics may enhance the potential for volume depletion. Monitor for signs and symptoms.
Lithium: Concomitant use with lithium may decrease serum lithium concentrations. Monitor more frequently during JARDIANCE initiation and dosage changes.
USE IN SPECIAL POPULATIONS
Pregnancy: JARDIANCE is not recommended during the second and third trimesters.
Lactation: JARDIANCE is not recommended while breastfeeding.
Geriatric Use: JARDIANCE is expected to have diminished glycemic efficacy in elderly patients with renal impairment. Assess renal function more frequently in elderly patients. The incidence of volume depletion-related adverse reactions and urinary tract infections increased in T2D patients ≥75 years treated with empagliflozin.
INDICATIONS AND LIMITATIONS OF USE
JARDIANCE is indicated:
- to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure
- to reduce the risk of sustained decline in eGFR, end-stage kidney disease, cardiovascular death, and hospitalization in adults with chronic kidney disease at risk of progression
- to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease
- as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus
JARDIANCE is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. It may increase their risk of diabetic ketoacidosis.
JARDIANCE is not recommended for use to improve glycemic control in patients with type 2 diabetes mellitus with an eGFR <30 mL/min/1.73 m2. JARDIANCE is likely to be ineffective in this setting based upon its mechanism of action.
JARDIANCE is not recommended for the treatment of chronic kidney disease in patients with polycystic kidney disease or patients requiring or with a recent history of intravenous immunosuppressive therapy or greater than 45 mg of prednisone or equivalent for kidney disease. JARDIANCE is not expected to be effective in these populations.
Please see Prescribing Information and Medication Guide for JARDIANCE.
CL-JAR-100162 09.21.2023
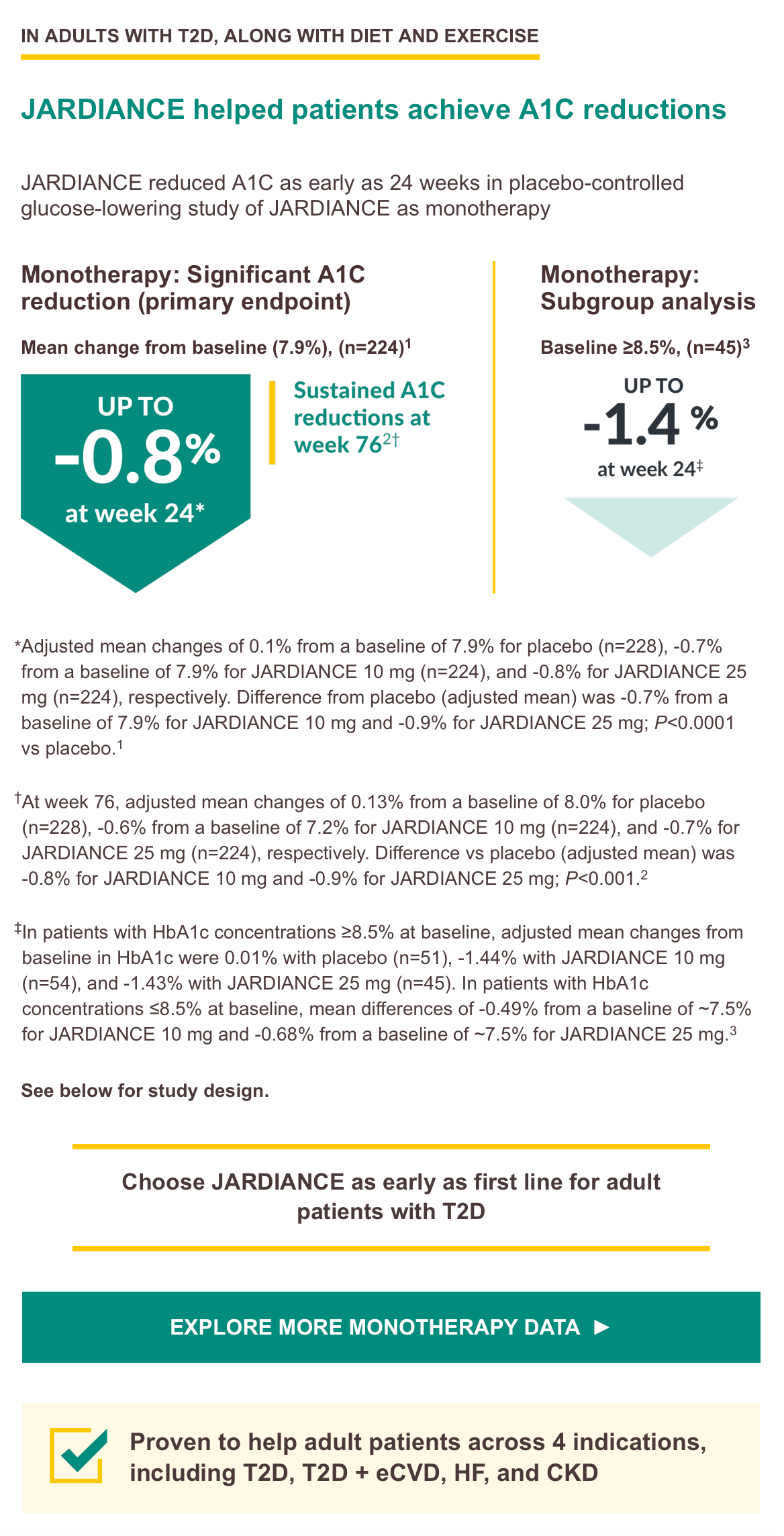
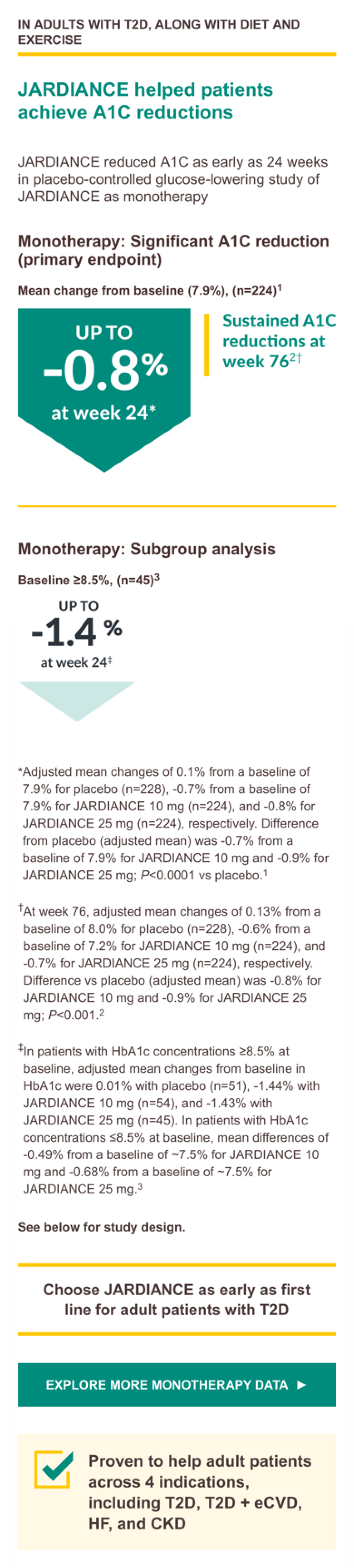
Monotherapy trial design: A Phase III, randomized, double-blind, placebo controlled, parallel-group efficacy and safety study of JARDIANCE administered orally once daily over 24 weeks in treatment-naïve patients with type 2 diabetes mellitus with insufficient glycemic control. A total of 676 treated patients received placebo (n = 228), JARDIANCE 10 mg (n = 224), or JARDIANCE 25 mg (n = 224). The primary endpoint was A1C change from baseline. Weight change and blood pressure change from baseline were secondary endpoints.3
CKD=chronic kidney disease; eCVD=established cardiovascular disease; eGFR=estimated glomerular filtration rate; HF=heart failure; IV=intravenous; T1D=type 1 diabetes; T2D=type 2 diabetes.
References: 1. Data on file. Boehringer Ingelheim Pharmaceuticals, Inc. 2. Roden M, Merker L, Christiansen AV, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naïve patients with type 2 diabetes: a double-blind extension of a phase III randomized controlled trial. Cardiovasc Diabetol. 2015;14:154. 3. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-210.


