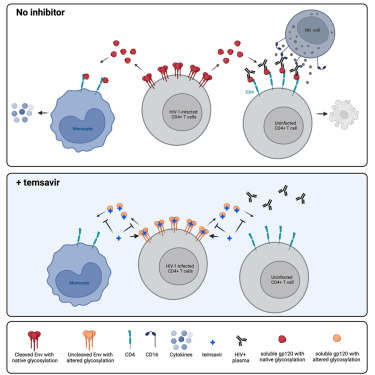
Temsavir blocks the immunomodulatory activities of HIV-1 soluble gp120
Richard et al. provide extensive evidence suggesting that treatment with the HIV-1 attachment inhibitor temsavir can have beneficial effects that extend beyond viral neutralization, notably by preventing elimination of uninfected...
Conclusions/Relevance: Temsavir prevents shed gp120 from interacting with uninfected bystander CD4+ cells, protecting them from ADCC responses and preventing a cytokine burst. Mechanistically, this depends on temsavir’s capacity to prevent soluble gp120-CD4 interaction, to reduce gp120 shedding, and to alter gp120 antigenicity. This...

Fostemsavir and ethinyl estradiol drug interaction: Clinical recommendations for co-administration - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36372442/
Applying the results of Study 206279 and other relevant ARV-contraceptive studies, we recommend that when co-administering fostemsavir with combined oral contraceptives (COCs) and other oestrogen-based therapies, EE dose should not...
Conclusions: Applying the results of Study 206279 and other relevant ARV-contraceptive studies, we recommend that when co-administering fostemsavir with combined oral contraceptives (COCs) and other oestrogen-based therapies, EE dose should not exceed 30 μg or equivalent, and caution is advised in the case of individuals with risk factors...

Study protocol for an efficacy trial of the "PrEP for Health" intervention to increase HIV PrEP use among people who inject drugs - BMC Public Health
Source : https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-15429-w
Background HIV incidence has recently increased among people who inject drugs (PWID) across the United States, with outbreaks occurring in states with long-standing syringe service programs (SSPs) including Massachusetts (MA)....
Conclusions/Relevance: In this study, we are evaluating the efficacy of the “PrEP for Health” intervention. If efficacious, findings from our implementation evaluation could help guide its dissemination to diverse SSPs and possibly other community-based settings accessed by this population.

Evaluating the efficacy of an online, family-based intervention to promote adolescent sexual health: a study protocol for a randomized controlled trial - Trials
Source : https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-023-07205-3
Background Adolescents in the U.S. experience significant negative sexual health outcomes, representing a public health priority in the U.S. Research shows that while parents play an influential role in shaping...
Discussion: The proposed evaluation and analysis of the FTT+ intervention will address gaps in the current cadre of parent-based programs. If efficacious, FTT+ would represent a model for scale-up and adoption of parent-based approaches designed to address adolescent sexual health in the U.S.

Health Economics Research on Non-surgical Biomedical HIV Prevention: Identifying Gaps and Proposing a Way Forward - PharmacoEconomics
Source : https://link.springer.com/article/10.1007/s40273-022-01231-w
Background and Objective Although HIV prevention science has advanced over the last four decades, evidence suggests that prevention technologies do not always reach their full potential. Critical health economics evidence...
Conclusions: Despite a large body of health economics evidence on non-surgical biomedical HIV prevention technologies, important gaps in the scope of evidence and methodology remain. To ensure that high-quality research influences key decision-making junctures and facilitates the delivery of prevention products in a way that maximises impact,...

