
Exploring current nutritional programming and resources available to people living with HIV/AIDs in Canada: a scoping review protocol - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36081361/
The following databases will be searched: MEDLINE (EBSCO), CINAHL (EBSCO), Academic Search Premier (EBSCO), Social Services Abstracts (ProQuest), and Scopus (Elsevier). Types of gray literature eligible for review include reports...
Relevance: The objective of this scoping review is to map the current literature and resources available on nutrition and foods programming for people living with HIV/AIDS in Canada. This review is phase one of a four-phase, provincially funded project called FoodNOW (Food to eNhance Our Wellness) focused on nutritional assessment of people...
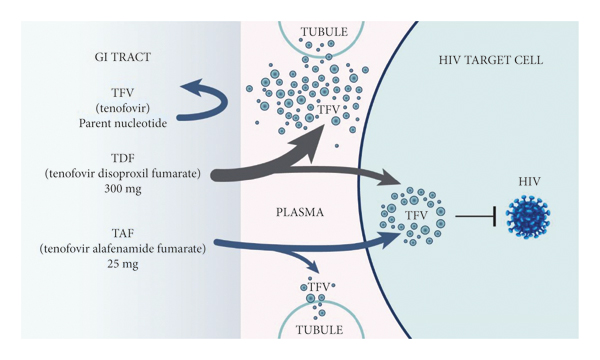
Clinical Considerations in the Selection of Preexposure Prophylaxis for HIV Prevention in Canada
Source : https://www.hindawi.com/journals/cjidmm/2022/3913439/
Clinical Considerations in the Selection of Preexposure Prophylaxis for HIV Prevention in Canada: According to the Public Health Agency of Canada, approximately 62,050 people were living with HIV in Canada...
Conclusions: PrEP is effective for many populations, including men who have sex with men, transgender women, heterosexuals with partners living with HIV, and people who use drugs. While there is fewer data reported on the efficacy of FTC/TAF to date, recent clinical trials have demonstrated noninferiority of FTC/TAF in comparison to FTC/TDF....

Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021 - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/35338549/
1 CHIP, Center of Excellence for Health, Immunity and Infections, Section 2100, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark. 2 Department of Infectious Diseases 144, Hvidovre University Hospital, Copenhagen, Denmark. 3...
Conclusions: In 2021, the EACS Guidelines were updated extensively and broadened to include new sections. The recommendations are available as a free app, in interactive web format and as an online pdf.

Temsavir Treatment of HIV-1-Infected Cells Decreases Envelope Glycoprotein Recognition by Broadly Neutralizing Antibodies
Source : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9239219/
mBio. 2022 May-Jun; 13(3): e00577-22. Marianne Boutin, a , b Dani Vézina, a Shilei Ding, a Jérémie Prévost, a , b Annemarie Laumaea, a , b Lorie Marchitto, a ,...
Importance: FDA-approved fostemsavir, the prodrug for the active moiety small molecule temsavir (GSK 2616713 [formally BMS-626529]), acts as an attachment inhibitor by targeting the HIV-1 envelope (Env) and preventing CD4 interaction. Temsavir also stabilizes Env in its “closed,” functional state 1 conformation, which represents an ideal...

Week 96 Genotypic and Phenotypic Results of the Fostemsavir Phase 3 BRIGHTE Study in Heavily Treatment-Experienced Adults Living with Multidrug-Resistant HIV-1
Source : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9211436/
Margaret Gartland, a Pedro Cahn, b Edwin DeJesus, c Ricardo Sobhie Diaz, d Robert Grossberg, e Michael Kozal, f Princy Kumar, g Jean-Michel Molina, h Fernando Mendo Urbina, i Marcia...
Conclusion/Relevance: In the phase 3 BRIGHTE study in heavily treatment-experienced adults with multidrug-resistant HIV-1, fostemsavir plus optimized background therapy (OBT) resulted in sustained rates of virologic suppression through 96 weeks.

